- Home page
- Fragments and Targeted libraries
- Macrocycles
UORSY Macrocycles
Macrocyclic compounds have distinguishing structural and physical-chemical properties beyond rule of five (bRo5).1 The unique features of macrocycles allow their application as inhibitors of challenging targets such as proteins with flat featureless surfaces or protein-protein interactions that are hardly tractable by small molecules.2,3
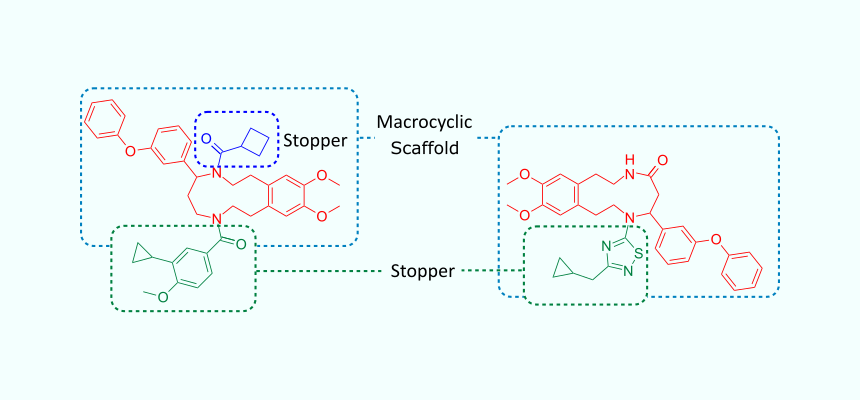
For a range of therapeutic targets, macrocycles could be isolated from natural sources. However, the variety of macrocyclic natural products is limited, thus synthetic approaches to macrocycles are needed. To address the issue of availability of diverse macrocyclic molecules, we have developed UORSY Feasible Macrocyclic Scaffold Library (FMSL). Chemically, the design of FMSL relies on the availability of reagents for both, 1) macrocyclic scaffold synthesis (by a synthetic chemist, based on our in-house experience), and 2) derivatization of the scaffold moieties with stoppers (using parallel synthesis approach). The macrocyclic scaffolds have one or two points of variation affording a highly diverse library.
For more information, please contact us at screenlibs@uorsy.com
1 Doak, B. C.; Over, B.; Giordanetto, F.; Kihlberg, J. Chem. Biol. 2014, 21 (9), 1115–1142.
2 Heinis, C. Nat. Chem. Biol. 2014, 10 (9), 696–698.
3 Driggers, E. M.; Hale, S. P.; Lee, J.; Terrett, N. K. Nat. Rev. Drug Discov. 2008, 7 (7), 608–624.
Ask your questions!
Fill in the form to ask a question.