- Home page
- Fragments and Targeted libraries
- Covalent fragments
UORSY Covalent Fragments
Numerous approved drugs and drug candidates possess covalent mechanism of action and the interest for finding novel entities acted as covalent inhibitors has significantly increased.1 As a result, the need for powerful tools for characterizing and optimizing covalent modifiers has aroused.
For creating our library of covalent fragments, we performed filtering based on fragment-like physicochemical profiles including molecules with certain functional groups or warheads.
Chemical classes included: acrylates and their active analogues; epoxides, lactones, lactams; sulfonyl fluorides; 2-chloropyridines; α,β-unsaturated sulfones and sulfonamides; activated cyano groups; aliphatic thiols; acetals and ketals.
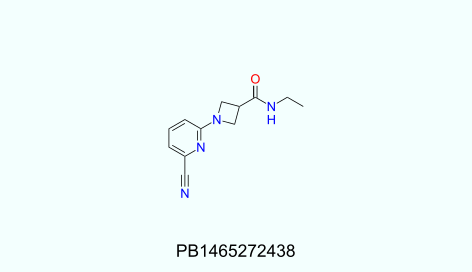
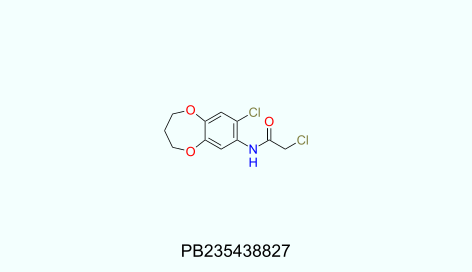
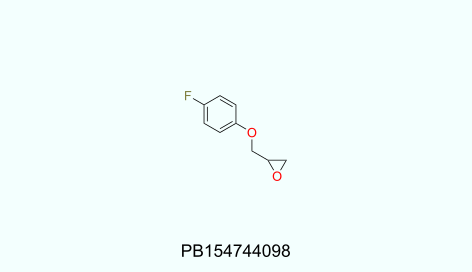
Physicochemical profiles of UORSY covalent fragments library:
130<MW<320; 1<HbA<5; 0<HbD<3; -1.8<logP<4; 0<RotBonds<4; TPSA<95.
UORSY covalent fragments library is available in stock and could be delivered within 2 weeks in any customer-preferred format: as powders, dry films or DMSO solutions formatted in vials, 96 or 384-well plates. All compounds have a minimum purity of 90% assessed by 1H NMR; analytical data is provided.
For more information, please contact us at screenlibs@uorsy.com
1 Mah, R.; Thomas, J. R.; Shafer, C. M. Drug Discovery Considerations in the Development of Covalent Inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 33–39
Ask your questions!
Fill in the form to ask a question.